Background.Blinatumomab is a monoclonal BITe antibody recently introduced for the treatment of relapsed and refractory (r\r) B-ALL. Response rate reaches 65-85% in different subgroups of patients but currently there is no reliable methods to predict response. Using results of the analysis of the efficacy of blinatumomab therapy in heterogenic cohort r/r B-ALL patients, including adults and children, we built a mathematical model to predict response to this antibody.
Patients and methods.A total of 128 pts with r\r B-ALL were included in the analysis, 52 children and 76 adults. Median age was 19 years (1-52). Hematological relapse (hr/r) and persistent measurable residual disease - molecular relapse (mr/r) were in 54% and 46% patients, respectively. Significant predictors of response in the multivariate analysis were used in the modeling for assessment of the probability of response to blinatumomab therapy. For validation of adjusted logistic regression parameters a k-fold cross-validation was performed. Confidence intervals for the model efficiency scores were calculated.
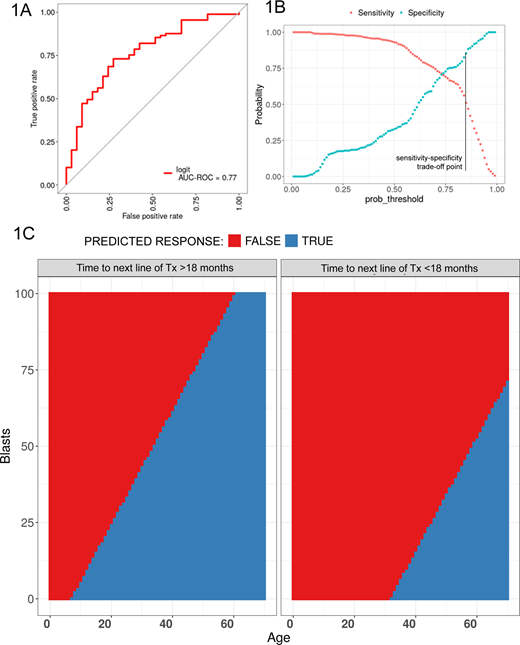
Results. The response rate to blinatumomab in the study cohort was 75%. In the univariate and multivariate analysis the following parameters had the highest significance for response: patient age (OR=12,06, 95% CI 3,6-40, p<0,001), short mean time (less 18 months) to next line of treatment (OR=0,17, 95% CI 0,05-0,5, p=0,002) and bone marrow blast percentage (OR=0,06, 95% CI 0,01-0,2, p<0,001). The resulting ROC-curve for the regression model is given in figure 1A. The sensitivity/specificity trade-off depending on a decision threshold level in given in figure 1B. For the final classification model for predicting response to blinatumomab therapy in pts r/r B-ALL decision-making threshold was adjusted for obtaining maximum specificity- 0,85, sensitivity -0,5 (i.e. select the patients who will very likely respond). Using this model, the following guiding maps were calculated (figure 1C). The following confusion matrix was obtained when applying the resulting model to the full database: the specificity was 90% (ratio 30/3) and sensitivity was 50% (ratio 44/45). From the confusion matrix the following confidential internals for sensitivity and specificity were estimated: sensitivity - point estimation= 0,51 (95% CI 0,4-0,61), specificity - point estimation= 0.91 (95% CI 0,76-0,98).
Conclusions.The possibility of response to blinatumomab therapy depends on patient's age, the bone marrow blast percentage and early relapse. The model predicts the response to blinatumomab therapy in patients with r/r B-ALL with high specificity and reasonable sensitivity and will be able to optimize the use of this very expensive therapy. Based on the model high probability of response in case of short time to next line of therapy is observed only for adult patients with low-to-moderate blast count, while in patients with long time to next line of therapy broader patient population has a high probability of response, including young children and patients with high blast count. Verification of this model in a larger multicenter study is required to confirm this hypothesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal